
Our attempt was to synthesize a material for the sensor, selective to only ammonia gas using Nano-composites of conducting polymer (Polyaniline, Polypyrrole) and two morphologies (spherical and rod) of anisotropic gold coated silica core-shell nanoparticles. Gas sensing properties of these materials depend on chemical composition, morphology, and method of preparation. Gold as a bulk does not interact with gases but its nanoparticles and composites show excellent gas sensing properties. Composites of conducting polymer and core-shell Gold-metal oxide nanoparticles prove to be the promising material for gas sensing. Various types of ammonia sensors are available but they subject to limitations. But it is irritating, inflammable and extremely hazardous in concentrated form.
It widely used in chemical industries, fertilizers, a precursor to nitrogenous compound, textiles, refrigeration etc. It plays a wide role in biological processes and physiology. The core-shell particle also was characterized by TGA, DLS, SEM and BET.Ammonia is one of the essential components of life formation. The overall size and shell thickness can be controlled by varying the reactant concentration both the core and shell material. The core surface was modified with CTAB miceller solution to make the coating favorable. The core shell particles were confirmed by DLS measurement and UV spectroscopy. The core sulfur particles were coated with AgBr to from core-shell particles in aqueous medium. The particle growth rate also depends on the surfactant concentration, with increase surfactant concentration growth rate decreases, and become constant near to its CMC value. The presence of surfactants creates a charged surface layer on the hydrophobic sulfur particle after adsorption and ultimately reduces the growth rate and agglomeration. Schwarb, Ryan Evan, Synthesis and characterization of reactive core-shell nanoparticles (2012). The increase in temperature and reactant concentration increases the size of the particle. Those parameters increase the diffusion rate show higher growth rate. The growth and agglomeration rate are depend on some reaction parameters, like temperature, reactant concentration, presence of different surfactants, and also on surfactant concentration. Actually, as reaction rate is very fast the growth rate is mainly depends on the diffusion of newborn particle from the bulk phase to surface of the nuclei. The protonization of the tertiary amine of the DEAEMA monomer (pKb 7.0 - 7.3) causes the PDEAEMA cores to experience a significant increase in particle size near neutral pHs. For this reaction nucleation is very fast so particle size is mainly controlled by particle growth and agglomeration. Core-shell nanoparticles are synthesized using a 2-stage surfactant-free emulsion polymerization. Sulfur obtained by this method was orthorhombic or -sulfur with S8 structureĪs the sulfur nanoparticles are formed by a precipitation reaction, therefore, the overall process can be subdivided in to three steps: nucleation, growth, and particle agglomeration. However, in CTAB solution, we got lower size particles in a certain reactant concentration range, and we got lowest 30 nm particles in this medium. Anionic surfactant SDBS is more efficient for controlling the uniform size in both the acids medium. The size reducing ability is not same for all the surfactants, depends on the types of surfactant. The effect of different surfactants (TX-100, CTAB, SDBS, and SDS) on particle size was also studied and found the surfactant can significantly reduce the particle size with out changing the shape. The size of the particles is also depends on the reactant concentration and acid to reactant ratio. Magnetic iron oxide core-shell nanoparticles, coated with a protective. We used both inorganic and organic acids, the results show organic acid gives smaller size of sulfur particles. Independent Project in Chemistry Bachelor thesis 15 hec First cycle,G2E. The sulfur nanoparticles were preparation by well known acid catalyzed precipitation reaction from sodium thiosulphate in presence of surfactants.
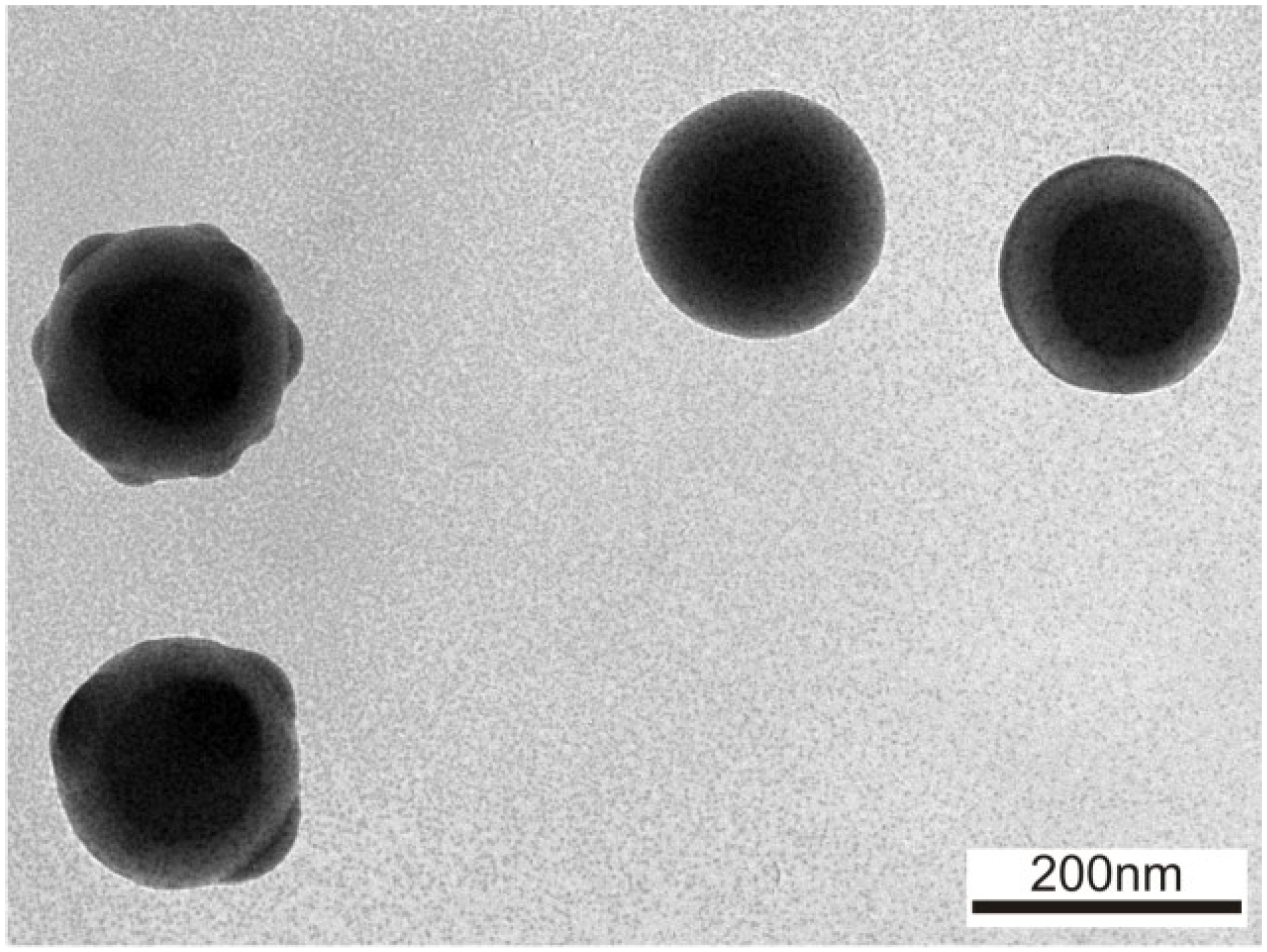
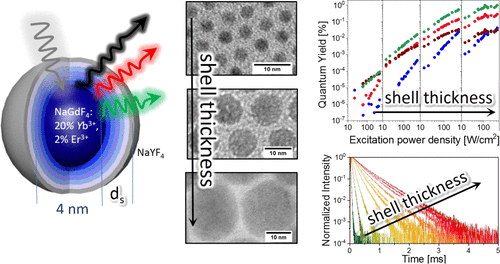
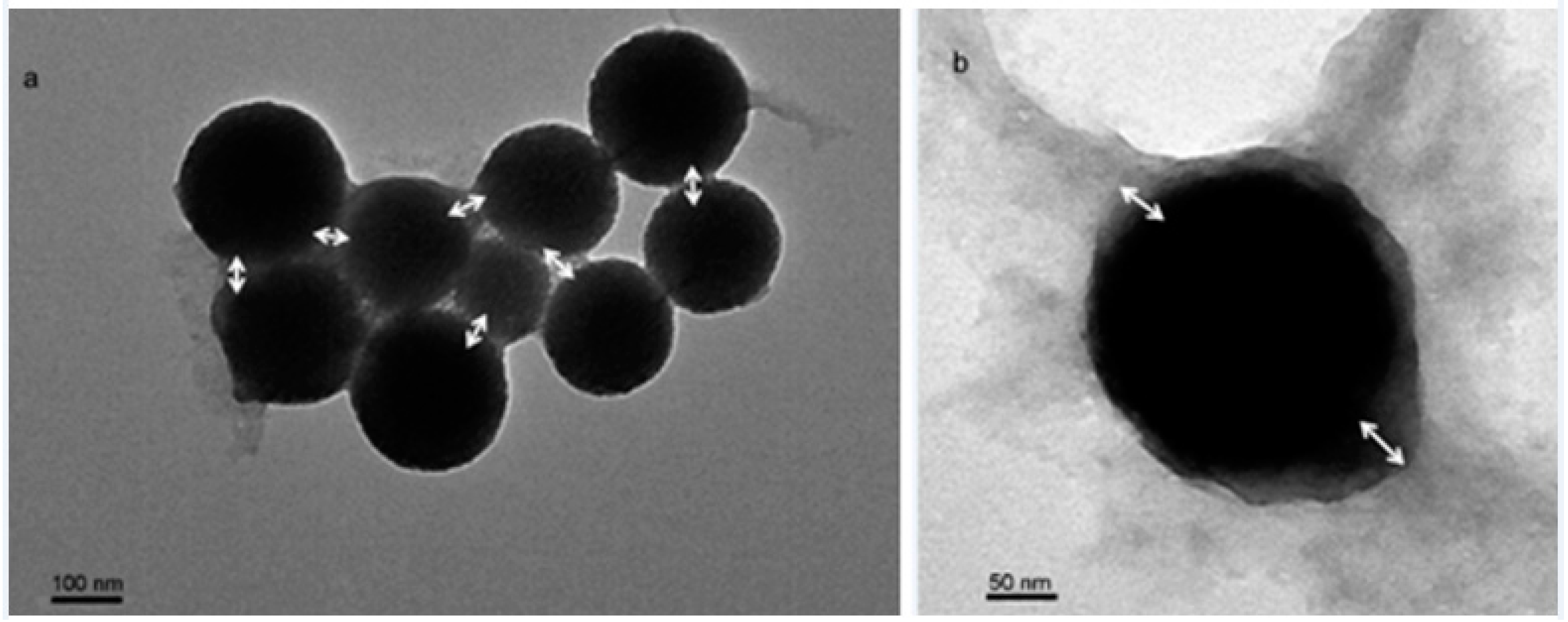
Finally to coat the core with AgBr in surfactant assisted medium. The main objective of this project is to study the effects of different parameters like types of acids, surfactants, reactant concentration, temperature, and sonication on core particle size and growth rate. In this report we studied the preparation and growth kinetics of sulfur nanoparticles as a core in aqueous surfactant medium and then the particle was coated with AgBr. They also show new properties than the single materials when coated one material with other. Core–shell nanoparticles are gaining lots of importance recently due to their exciting applications in different fields like biomedical, pharmaceutical, electronics, catalysis etc.


 0 kommentar(er)
0 kommentar(er)
